Salt chlorine generators have rapidly become a mainstream sanitizing system for new pools. And why not? By automating the addition of chlorine to a pool they eliminate the human errors that can cause drops in sanitizer level and cause algae blooms. They obviate the need for on-site buckets of chlorine - always a concern for homeowners with children. And they require less work.
However, a disconnect remains between the homeowner's perception of salt pools - often fostered by builders who are primarily focused on the sale - and the reality of their maintenance, particularly as it relates to staining.
"Customers tend to think that once they have a salt pool system installed, they can just let it go," says Kevin Bean, partner at Argus Pool Supplies, Corona, Calif. "That's really just the beginning of an education process, I've found. They're often surprised to learn that they have to do more than what the builder told them."
In short, homeowners often come to believe that with the addition of a salt system their burden of care is at an end, but the fact is that staining happens in any pool, regardless of sanitizer system, and certain kinds of stains are caused by elements of a salt system.
Metal stains due to galvanic corrosion are an example, and the incidence of this problem has grown alongside the rise in popularity of salt systems nationwide.
In the galvanic corrosion process, metals from the "noble-cathodic" end of the galvanic chart (see table) steal electrons from metals at the other "least-noble-anodic" end and cause them to deteriorate in the pool, causing stains or solid, insoluble deposits. The unique conditions in a salt chlorine pool (as opposed to a traditionally sanitized pool) encourage this type of corrosion.
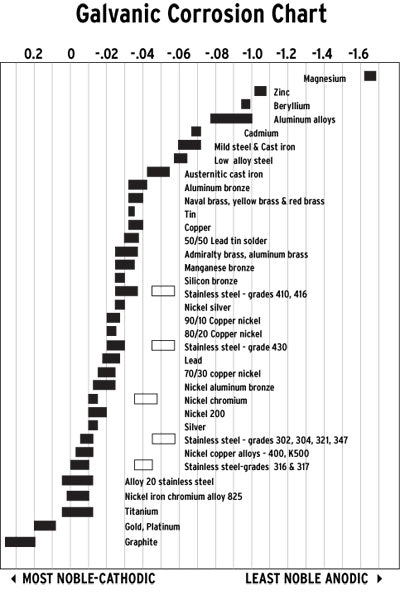
This galvanic chart ranks dissimilar metals, cathodic to anodic. The farther apart metals are from each other on this chart, from left to right, the more severe the galvanic activity. For example, there is more galvanic activity between titanium and zinc than titanium and Copper. The more cathodic metal will attack the more anodic.
In the case of salt chlorine pools, the metal doing the stealing is typically the titanium plate in the salt chlorine generator cell, and the metal getting ripped off is copper from the heat exchanger or other copper sources, iron from steel screws, and other anodic metals present in pool equipment and fittings.
What the pool professional is likely to observe as a result, notes Terry Arko, product specialist for SeaKlear Pool and Spa Products based in Bothell, Wash., includes:
- black flecks on pool bottom
- black staining on ladders and light rings
- reoccurring stains and discoloration on light rings around steps or rails and discolored water
- purple haze and debris in pool water.
"The simple solution to solve this problem," Arko says, "is to find another less-noble metal to use as a sacrificial anode that corrodes but doesn't cause staining."
Translated, that means if you can put something benign like zinc, which is more susceptible to galvanic corrosion, in the water, the zinc will "sacrifice" itself for the copper. That is to say, the titanium will steal electrons from the zinc and cause it to degrade, leaving the copper alone. Zinc dissolved in pool water doesn't cause staining; so the problem is solved.
Fred Johnson, operations manager, Blue Water Pool & Repair Corporation, Orlando, Fla., uses zinc in his pools with good result. "It's the best thing you can do to prevent this type of problem," he says. "You can use it as an additive, or you can hang it in the circulation of the pool - that works very well."
"If the water is already discolored from copper, it is advised to use a metal removal product along with the zinc to remove the current discoloration and prevent reoccurrence," Arko adds. "Most metal products on the market tend to be phosphate based and this, too, can cause problems in a salt chlorine generator. When selecting a metal product use a phosphate-free product."
Bad Salt
Another cause of staining particular to salt chlorine pools is the main ingredient itself - salt. There is a common perception that any old salt thrown into the pool will happily break down into free chlorine in the generator, which is essentially true, but few consider what may accompany the salt in the bag.
"It's a huge thing that I talk to people all the time about," says Johnson. "They get salt from Costco and Lowes - it's cheap but not refined enough. Even though it says it's 99 percent pure, that 1 percent can cause staining."
For example, in a 22,000-gallon pool on startup, 530 pounds of salt should be added to reach proper concentration. If that salt is 99 percent pure (or 1 percent impure), 5.3 pounds of impurities have been added to the pool, easily enough to cause a stain under the right conditions.
The salt problem is also related to its application, notes Bob Harper, general manager of Pristiva, a salt manufacturer owned by Compass Minerals. Salt that is added and falls undissolved to the bottom of the pool can create a surface problem very similar in appearance to staining.
"Salt lying on the bottom or clinging to the side of a pool creates a localized area of higher ionic concentration," he says, "which can exacerbate staining and cause calcium to come to the surface of the concrete - efflorescence - a light-colored stain on the side of concrete.
"This is something the industry is paying more attention to. Sometimes you see it on cementitious surfaces. It's typically more common during the curing of the material. As the salt lays on the surface of the pool, the ionic strength in that area goes way up, which causes a migration of calcium salts to pass through the cement itself and bring it to the surface. And that can cause a discoloration. This is especially a problem on colored plaster finishes. That efflorescence will create a lightening of the plaster as those salts are drawn to the surface of it."
Aggressive brushing and simply not allowing the salt to lie on the bottom of the pool can avoid this. And it helps to both apply salt to the deep end where there's more volume to dissolve the salt and to make sure the pump is running to keep the water moving. Still, Harper adds, salts that dissolve faster ameliorate the problem, as service personnel and homeowners are not always diligent about ensuring all added salt is dissolved.
Breakdown
A third staining problem unique to the salt system is the breakdown of sequestering agents, which can be caused by the salt generator cell. These sequestering agents are relied upon to hold metals in solution, but the extreme conditions encountered within the salt generator cell can degrade them, causing them to release metals in a stain.
At some points inside the salt chlorine generator, chlorine concentrations can reach 50 ppm (the normal recommended level for pool water is a maximum of 4 ppm), pH can be either close to 14 or 0 (normal levels between 7.2 and 7.8) and temperatures can exceed 120 degrees, conditions destructive to the chemistry of traditional pool additives.
Unsuspecting pool techs may assume the effective life of these products is unchanged in a salt chlorine pool, Harper says. "For instance, often these products are applied monthly. But I think it's safe to say that most of those products don't last more than two or three weeks in that environment."
Even other chemicals intended for entirely different problems can cause problems if not designed for the salt chlorine generator environment, Harper adds.
"These are things like copper-containing algaecides - there are many different types, some are fairly stable - by that I mean they will hold up in high-chlorine environments such as you have in a salt generator cell - and some are not, depending on the chemistry," says Harper.
He stresses the importance of understanding how a salt chlorine generator works and how it affects pool care.
While the salt chlorine revolution has improved pool maintenance in the industry in general, it has also introduced new challenges - galvanic corrosion, salt quality and application issues, and chemical additive stability - that must be recognized and addressed to fully realize its benefits.
Targeted Stain Removal
Here's an interesting time saver for cleaning up small stains at the bottom of a pool without draining, offered by Kevin Bean, partner at Argus Pool Supplies, Corona, Calif.:
"It's just for small stains, like if somebody accidently drops a nail down in the pool leaving a rust stain or something like that. You just stick an 8-foot stick of ¾-inch PVC pipe down there and funnel your acid down the pipe on each spot. The acid pushes right down to the stain and removes it.
"It's a quick and easy way to get rid of some stains cheaply without draining the whole pool, and it works great. I've done it many times. Just don't forget to add some soda ash when you're done to neutralize the acid, but you can calculate exactly how much to add based on the exact amount of acid you added."
- S.W.
Comments or thoughts on this article? Please e-mail [email protected].